The combination of HCOOCH (methyl formate), CH₂ (methylene group), and H₂O (water) represents an interesting case in organic chemistry. Each of these substances plays a distinct role in synthesis, hydrolysis, and catalytic reactions. While they may not always react directly under standard conditions, their interaction under specific environments yields useful intermediates or end products.
Let’s break down each component:
-
HCOOCH is methyl formate, an ester formed from formic acid and methanol.
-
CH₂ typically refers to the methylene group, a reactive intermediate often introduced as a carbene (:CH₂) or stabilized form.
-
H₂O is water, a universal solvent and a reactant in hydrolysis and condensation reactions.
Understanding how these compounds interact can help chemists optimize reaction conditions for synthetic goals, from pharmaceuticals to polymers.
Properties of Methyl Formate (HCOOCH)
Methyl formate is a volatile organic compound with a fruity odor. It is used as:
-
A solvent in industrial applications
-
An intermediate in the manufacture of formamide and formic acid
-
A reagent in esterification and hydrolysis
Key properties:
-
Boiling point: 31.5°C
-
Molecular weight: 60.05 g/mol
-
Density: 0.97 g/cm³
Its reactivity is largely due to the ester bond, which is susceptible to nucleophilic attack, particularly by water in hydrolysis or base-catalyzed reactions.
Reactivity of CH₂ (Methylene Group)
CH₂ as a stand-alone group is extremely reactive. It generally exists as:
-
Methylene carbene (:CH₂), a divalent carbon intermediate
-
Stabilized methylene in the form of CH₂Cl₂ (dichloromethane) or CH₂= (part of a double bond)
In synthetic chemistry, :CH₂ is generated in situ from compounds like diazomethane or methylene iodide with zinc-copper couple. These species are often used in:
-
Cyclopropanation reactions
-
Ylide-based transformations
-
C–C bond formation
H₂O as a Reactant in Organic Chemistry
Water is a vital component in many organic reactions:
-
Hydrolysis of esters and amides
-
Condensation and dehydration reactions
-
Acts as a nucleophile in acid/base catalysis
In the context of HCOOCH, H₂O often participates in hydrolysis to yield methanol and formic acid, especially under acidic or basic conditions.
Possible Reactions Between HCOOCH, CH₂, and H₂O
Although no direct, simple three-component reaction between methyl formate, methylene, and water is standard in textbooks, several synthetic pathways may involve combinations of these species. Let’s explore a few possibilities.
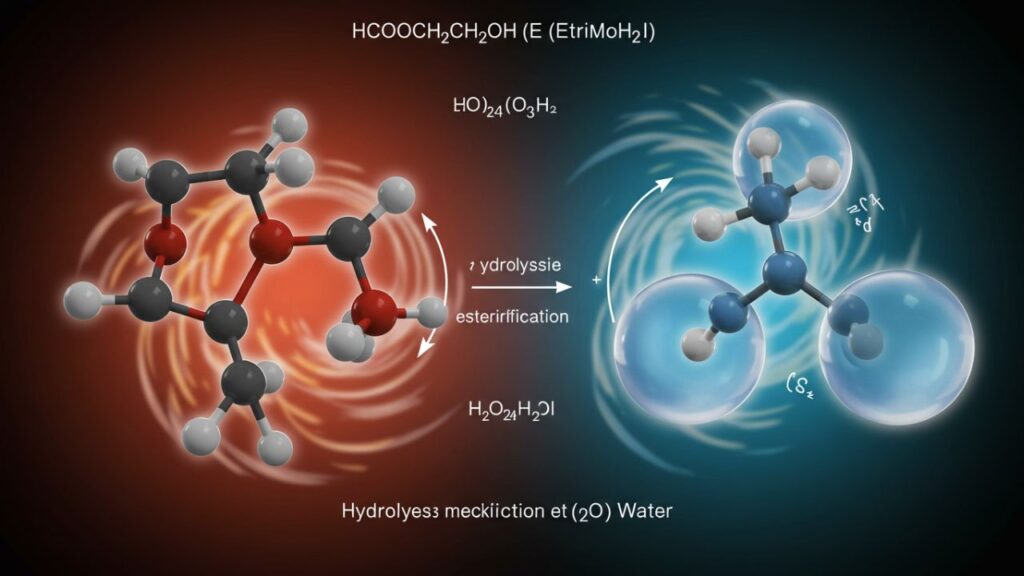
1. Hydrolysis of HCOOCH in the Presence of H₂O
Equation:
HCOOCH + H₂O → HCOOH + CH₃OH
Under acidic or basic conditions, methyl formate undergoes ester hydrolysis, producing formic acid and methanol. This is a well-known and straightforward reaction facilitated by either HCl or NaOH as catalysts.
2. Generation of CH₂ Carbenes for Organic Synthesis
CH₂ groups, introduced via diazomethane (CH₂N₂), can insert into C–H bonds or react with alkenes. In some experimental systems, methyl formate or its derivatives can participate in reactions with CH₂ sources, creating substituted methylene products.
Example Reaction:
RCH=CH₂ + :CH₂ → Cyclopropane derivative
While methyl formate doesn’t directly generate CH₂, reactions involving CH₂ insertion may affect methyl esters under certain catalytic systems.
3. Acid-Catalyzed Reaction of CH₂ with HCOOCH
With appropriate catalysts (like Lewis acids), CH₂ can act as a nucleophile or electrophile in reactions with esters. In theoretical or experimental settings, insertion reactions or ylide-mediated transformations can occur between CH₂ and HCOOCH analogs.
However, such reactions are highly dependent on conditions like:
-
Solvent system (water, ether, toluene)
-
Temperature
-
pH levels
-
Presence of metal catalysts (Cu, Zn, Rh)
4. Aqueous-Phase Reactions of Methylene Compounds
If a CH₂ source is water-compatible (e.g., diazomethane in aqueous suspension), it may form hydroxy-substituted methylene intermediates. These are short-lived and often decompose to form formaldehyde, methanol, or CO₂.
Applications in Organic Synthesis
The components HCOOCH, CH₂, and H₂O appear independently in many key reactions:
-
Methyl formate is a building block for heterocyclic synthesis and CO-releasing compounds.
-
CH₂ intermediates are used in polymer formation, fuel synthesis, and pharmaceuticals.
-
Water serves as a solvent in green chemistry, allowing reactions to proceed without hazardous organic solvents.
A deeper understanding of how these substances behave enables chemists to:
-
Design more sustainable synthetic routes
-
Avoid hazardous by-products
-
Enhance reaction selectivity and yield
Green Chemistry Implications
Using water as a solvent, and mild esters like methyl formate, aligns with the principles of green chemistry. Efforts are ongoing to:
-
Replace chlorinated solvents with methyl formate
-
Develop water-compatible methylene transfer reactions
-
Improve ester hydrolysis efficiency with recyclable catalysts
This reflects a broader shift toward eco-friendly and cost-effective processes in chemical manufacturing.
Experimental Considerations
When exploring reactions involving these compounds, chemists must consider:
-
Toxicity: Diazomethane (CH₂N₂) is highly toxic and explosive. Handle with extreme caution.
-
Volatility: Methyl formate is flammable and evaporates quickly; reactions must be conducted in fume hoods.
-
pH Sensitivity: Hydrolysis of esters is highly pH-dependent; buffers or acids are often required.
-
Catalysis: The use of transition metals or solid acid catalysts can dramatically alter product formation.
Research Frontiers
Ongoing studies are investigating:
-
One-pot syntheses combining esters and methylene donors
-
Photochemical reactions involving CH₂ and ester systems in water
-
Biocatalysis using enzymes to control ester breakdown or methylene insertion
-
Reactive intermediates like carbonyl ylides from HCOOCH and CH₂ analogs
These innovations aim to simplify reaction steps, reduce waste, and improve atom economy.
Final Thought
While a direct, textbook reaction involving all three components—HCOOCH, CH₂, and H₂O—may not be commonplace, the interplay between esters, reactive methylene species, and water is fundamental to many synthetic pathways. Understanding each component’s role allows chemists to design innovative, safe, and high-yielding reactions.
Whether in academic research or industrial application, the chemistry of HCOOCH, CH₂, and H₂O continues to evolve, with ongoing interest in greener, more efficient transformations.